David Geier, Vaccine Skeptic, Joins HHS To Analyze Vaccine Studies

Table of Contents
Geier's Background and History of Vaccine Criticism
Dr. Geier's past publications and public statements have consistently expressed skepticism regarding vaccine safety. He's been a vocal critic of various aspects of vaccine research and policy, often participating in debates and discussions that challenge the prevailing scientific consensus on vaccine efficacy and safety. His work has been cited by anti-vaccine groups, though he himself doesn’t explicitly identify as part of the anti-vaccine movement. [Insert links to relevant publications and articles here]. Keywords: vaccine controversy, anti-vaccine movement, vaccine side effects, scientific literature.
Specific Concerns Raised by Geier
Dr. Geier's concerns have focused on several key areas:
- Vaccine Ingredients: Concerns about the potential long-term effects of specific vaccine ingredients, such as thimerosal (a preservative).
- Long-Term Vaccine Effects: Questions about the possibility of long-term adverse events linked to vaccination, including potential links to autoimmune disorders.
- Correlation vs. Causation: Criticisms of studies that he believes fail to adequately address the difference between correlation and causation regarding vaccine administration and subsequent health issues.
- Vaccine Efficacy: Questions about the actual efficacy of certain vaccines and whether the benefits outweigh the potential risks for all individuals.
Keywords: vaccine ingredients, long-term vaccine effects, vaccine adverse events, vaccine efficacy.
Geier's Role at HHS and Potential Conflicts of Interest
The specifics of Dr. Geier's role within the HHS remain unclear [insert details about his position and responsibilities if available]. However, his history of vaccine skepticism raises serious concerns about potential conflicts of interest. His appointment could be perceived as undermining the HHS's commitment to promoting vaccine uptake and could impact the objectivity and transparency of vaccine research and policy decisions within the agency. The potential for bias in research funding decisions, policy recommendations, and public communication strategies presents a significant challenge. Keywords: conflict of interest, vaccine policy, government regulation, transparency in research.
Reactions from Public Health Officials and Experts
The appointment has been met with a mixed response. Some public health officials and experts have expressed serious concerns about Geier's appointment, citing his history of promoting vaccine skepticism and the potential for this to undermine public trust in vaccines. Others have argued that his perspective could offer valuable insights into the ongoing dialogue surrounding vaccine safety, emphasizing the importance of a diversity of views in scientific discourse. [Include quotes from relevant experts if available]. Keywords: public health response, expert opinion, vaccine mandates, vaccine hesitancy.
The Impact on Vaccine Research and Policy
Dr. Geier's presence within the HHS could significantly impact future vaccine research and policy. His views could influence funding decisions, shaping the direction of research efforts and potentially hindering research into the safety and efficacy of vaccines. Furthermore, his influence could affect the communication strategies employed by the HHS, potentially increasing vaccine hesitancy among the public. The potential impact on public trust in vaccines and the overall success of vaccination programs is a serious consideration. Keywords: vaccine development, vaccine regulation, public trust in science, healthcare policy.
Future Research Directions and Calls for Transparency
Addressing concerns about vaccine safety and efficacy requires rigorous, transparent, and unbiased research. Future research should prioritize:
- Large-scale, well-designed clinical trials.
- Comprehensive epidemiological studies to investigate potential long-term effects.
- Open and accessible data sharing to ensure transparency and reproducibility of results.
Maintaining scientific integrity and fostering public trust necessitates transparency in all aspects of vaccine research and policy-making. Keywords: clinical trials, epidemiological studies, data transparency, scientific integrity.
Conclusion: Analyzing the Implications of David Geier's HHS Role – A Call for Scrutiny
The appointment of Dr. David Geier to the HHS is a significant development with potentially far-reaching consequences. His history of vaccine skepticism raises serious concerns about conflicts of interest and the potential impact on vaccine research and public health policy. The need for transparency, objectivity, and rigorous scientific investigation in all aspects of vaccine research is paramount. We must remain vigilant and critically examine information related to vaccines, relying on evidence-based science from credible sources. Stay informed and continue to advocate for transparent and unbiased vaccine research. This appointment underscores the ongoing importance of critical thinking and informed consent when it comes to vaccination. Keywords: vaccine safety information, critical thinking, reliable sources, informed consent. Understanding the nuances of David Geier's role and his influence on vaccine skepticism within the HHS is crucial for shaping informed public health discourse.

Featured Posts
-
 Pne Ag Ad Hoc Mitteilung Gemaess Artikel 40 Absatz 1 Wp Hg
Apr 27, 2025
Pne Ag Ad Hoc Mitteilung Gemaess Artikel 40 Absatz 1 Wp Hg
Apr 27, 2025 -
 Discover Hidden Gems Free Movies And Shows On Kanopy
Apr 27, 2025
Discover Hidden Gems Free Movies And Shows On Kanopy
Apr 27, 2025 -
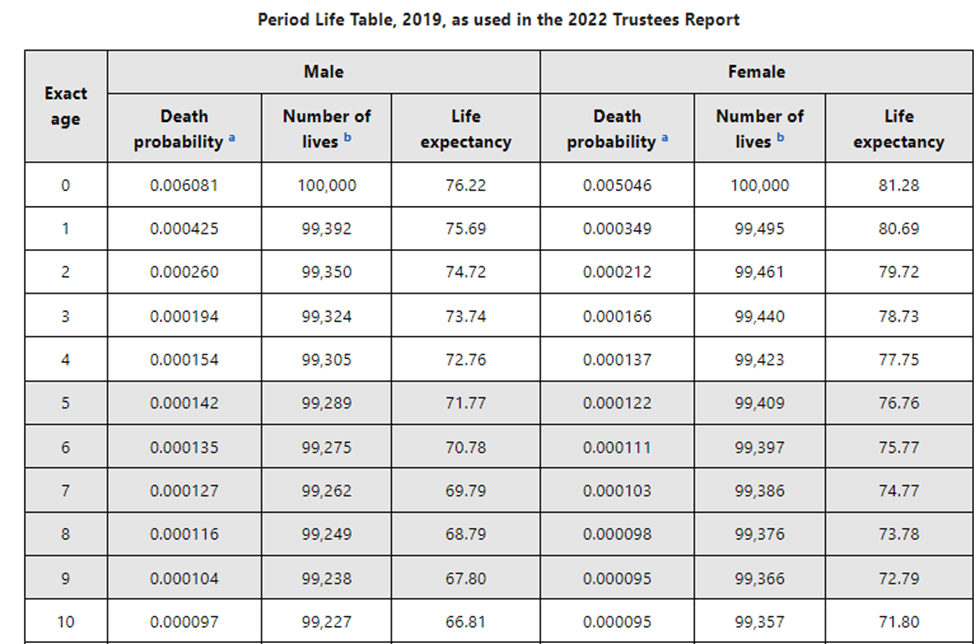 Grand National Pre 2025 Analysis Of Horse Mortality
Apr 27, 2025
Grand National Pre 2025 Analysis Of Horse Mortality
Apr 27, 2025 -
 The Cdcs New Vaccine Study Hire Fact Checking The Misinformation Controversy
Apr 27, 2025
The Cdcs New Vaccine Study Hire Fact Checking The Misinformation Controversy
Apr 27, 2025 -
 Ariana Grandes Style Evolution Hair Tattoos And Professional Insights
Apr 27, 2025
Ariana Grandes Style Evolution Hair Tattoos And Professional Insights
Apr 27, 2025
